Introduction
Nukleotidy are the fundamental building blocks of life. They form the structure of DNA and RNA, carry energy in the form of ATP, and regulate vital cellular processes. Understanding nukleotidy helps us uncover how genetic information is stored, transmitted, and expressed. In this guide, we explore their structure, types, functions, and applications in modern science.
1. What Are Nukleotidy?
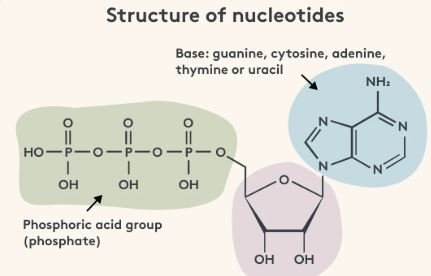
Nukleotidy (nucleotides in English) are organic molecules composed of three parts:
- A nitrogenous base
- A five-carbon sugar
- One or more phosphate groups
They are present in every living cell and play central roles in genetics, metabolism, and biochemistry. Without nukleotidy, life as we know it would not exist.
2. Structure of Nukleotidy
a) Nitrogenous Bases
The base gives each nukleotid its identity. There are two categories:
- Purines: Adenine (A) and Guanine (G)
- Pyrimidines: Cytosine (C), Thymine (T – found in DNA), and Uracil (U – found in RNA)
b) Sugar Component
- In RNA, the sugar is ribose.
- In DNA, the sugar is deoxyribose (lacking one oxygen atom compared to ribose).
c) Phosphate Group(s)
Nukleotidy may carry one, two, or three phosphate groups. These groups store chemical energy in high-energy bonds. Common forms include:
- AMP (adenosine monophosphate)
- ADP (adenosine diphosphate)
- ATP (adenosine triphosphate)
3. Types of Nukleotidy
Nukleotidy can be classified based on their sugar and base:
- Ribonukleotidy (RNA building blocks): A, U, G, C
- Deoxyribonukleotidy (DNA building blocks): A, T, G, C
Each combination allows for genetic diversity and proper encoding of biological information.
4. How Are Nukleotidy Formed?
Nukleotidy are synthesized by adding a phosphate group to a nukleosid (nucleoside). A nucleoside is made of a sugar and a base. When phosphorylated at the 5′ carbon of the sugar, it becomes a full nukleotid. Cells can build them from scratch (de novo pathway) or recycle them (salvage pathway).
5. Functions of Nukleotidy
a) Genetic Information Storage
Nukleotidy link together through phosphodiester bonds to form DNA and RNA chains. The sequence of nukleotidy encodes genes, which control protein synthesis and heredity.
b) Energy Transfer
ATP is often called the “energy currency” of the cell. It releases energy when phosphate bonds are broken, fueling muscle contraction, nerve impulses, and biochemical reactions.
c) Enzyme Cofactors
Nukleotidy are part of cofactors like NAD, NADP, FAD, FMN, and Coenzyme A. These molecules help enzymes function during respiration, metabolism, and biosynthesis.
d) Cell Signaling
Cyclic nukleotidy (cAMP and cGMP) act as “second messengers.” They transmit signals inside cells, regulating processes such as hormone response, metabolism, and gene expression.
e) DNA Replication and Repair
Complementary base pairing between nukleotidy (A–T and G–C) ensures accurate DNA replication and repair, maintaining genetic stability across generations.
6. Role of Nukleotidy in Molecular Biology
- PCR (Polymerase Chain Reaction): Uses dNTPs (deoxynukleotid triphosphates) as substrates for DNA synthesis.
- DNA Sequencing: Sanger sequencing employs ddNTPs (dideoxynukleotidy), which terminate DNA chains for accurate sequencing.
- Fluorescent Labeling: Modified nukleotidy are tagged with dyes or biotin to study DNA and RNA in research labs.
7. Medical and Biotechnological Importance
- Genetic Testing: Nukleotidy allow scientists to read genetic codes and identify mutations.
- Drug Design: Antiviral drugs like AZT mimic nukleotidy and interfere with viral replication.
- Nutritional Supplements: Some baby formulas and medical foods are enriched with nukleotidy to boost immune health and gut development.
- Cancer Research: Abnormal nukleotid metabolism is linked to tumor growth, making them targets for chemotherapy.
8. Summary Table of Nukleotidy Roles
| Function | Example Molecule | Role in Cells |
| Genetic storage | DNA/RNA | Encode genes |
| Energy transfer | ATP | Fuel reactions |
| Enzyme cofactors | NAD, CoA | Support enzymes |
| Signal transduction | cAMP, cGMP | Cell signaling |
| Research tools | dNTPs, ddNTPs | PCR, sequencing |
9. Why Are Nukleotidy Essential for Life?
Nukleotidy bridge multiple areas of biology:
- They preserve genetic memory through DNA.
- They provide energy for growth and survival.
- They allow communication between cells via signaling.
- They enable scientific discovery through biotechnology.
In short, nukleotidy represent the foundation of both natural and applied life sciences.
Conclusion
Nukleotidy are more than just chemical compounds. They are the key to understanding how life is structured, powered, and regulated. From storing DNA to fueling metabolism and enabling scientific breakthroughs, nukleotidy occupy a central role in biology. As research advances, the importance of these molecules continues to grow, making them a vital subject of study in genetics, medicine, and biotechnology.
Frequently Asked Questions (FAQ)
1. What is the difference between a nukleosid and a nukleotid?
- A nukleosid has a base and a sugar. A nukleotid has a base, a sugar, and phosphate group(s).
- ATP stores energy in its phosphate bonds. Breaking these bonds releases energy for cellular work.
2. Why is ATP considered the energy currency?
3. What does cAMP stand for?
- cAMP means cyclic adenosine monophosphate. It acts as a messenger in hormone signaling and metabolism.
4. Do nukleotidy exist only in DNA and RNA?
- No. Besides forming DNA and RNA, nukleotidy also act as cofactors, energy carriers, and signals in metabolism.
5. How are nukleotidy used in biotechnology?
- They are essential in PCR, DNA sequencing, genetic testing, and drug development.
Rea More: Jpost